Enzymatic conversions in highly concentrated reaction systems
Introduction
One of the most important enzymatic reactions that is carried out at an industrial scale is the enzymatic hydrolysis of starch (see figure 1). Currently, it is carried out at a substrate concentration of 35-40 w %1. Increasing the substrate concentration has several advantages: increased volumetric productivity and a higher α-amylase stability2-4. However, new reactor concepts need to be developed to handle liquids with a high viscosity and the influence of these conditions on the enzyme activity and volumetric productivity needs to be considered.

Figure 1: Scheme for starch-hydrolysis (abbreviations: α: α-amylase, ß: ß-amylase)
Aim of this study
The aim of this project is to investigate the fundamental processes that take place during the enzymatic hydrolysis of starch at highly concentrated reaction conditions leading to the desired product composition. The information gathered during this investigation will be used to develop and test suitable reactor concepts.
Effect of carbohydrates on activity measurements
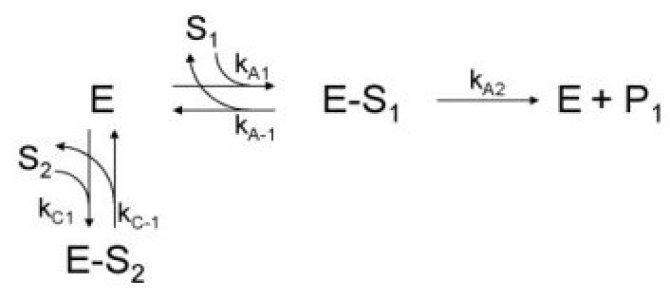
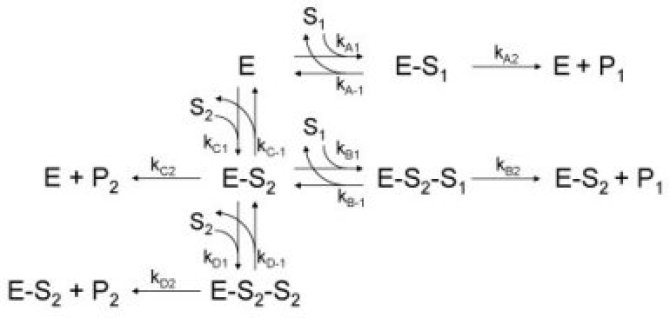
α-Amylase (1,4-a-D-glucanohydrolase, E.C. 3.2.1.1) is essential during the enzymatic hydrolysis of starch. This enzyme plays an important role in the liquefaction of starch and the subsequent saccharification where larger sugar chains are hydrolyzed and converted into smaller sugars5 (see figure 1). It is therefore required to preserve the highest α-amylase activity during operating conditions. A suitable assay procedure was chosen to determine the residual α-amylase activity under these operating conditions. We chose for the Ceralpha method6 that was developed by McCleary and Sheehan7,8 and now commercialized by Megazyme. This procedure is based on the hydrolysis of blocked p-nitrophenolmaltoheptaoside substrate and the subsequent release of p-nitrophenol. This is a rapid and accurate assay9 that can be used for samples with high background levels of small sugars. However, the effect of the presence of several small carbohydrates on the measurement of the α-amylase activity with the Ceralpha procedure was not determined before. We have investigated the effect of several carbohydrates over a broad concentration rangeb,c. A simple kinetic model based on substrate competition or substrate inhibition (see figure 2) was usedb to describe the effect soluble potato starch (see figure 3). This model was able to describe the decrease in α-amylase activity that was observed when the soluble starch concentration was increased.
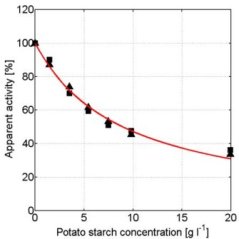
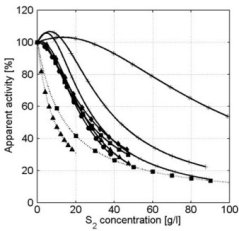
In the presence of small carbohydrates, a distinct maximum in the α-amylase activity vs. concentration curves was observed (e.g. glucose, see figure 4). In the α-amylase activity vs. concentration curves of maltose and maltotriose maxima were also observed. If the carbohydrate is larger than maltotriose, the maximum is no longer present (see for example maltotetraose, figure 5). At higher concentrations, all carbohydrates show a decreasing α-amylase activity at increasing carbohydrate concentrations (see figure 7).
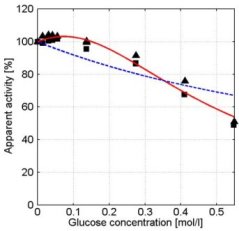
Figure 4: Apparent α-amylase activity towards blocked p-nitrophenyl maltoheptaoside as function of the D( )-glucose concentration. Experimental data obtained with duplicate experiments (experiment 1: squares experiment 2: triangles) The solid red line describes the model data based on the kinetic scheme in Figure 6 and the dashed blue line describes the model data based on the scheme in Figure 2. Conditions: CE = 1.1∙10-2 g∙l-1, T = 40°C, pH = 6.5. Without carbohydrates ([S2] = 0) the α-amylase activity is 100% which equals 1.51 ± 0.08µmol∙mg-1∙min-1.
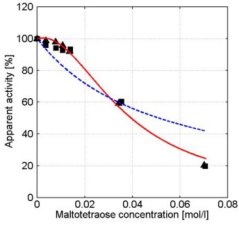
Figure 5: Apparent α-amylase activity towards blocked p-nitrophenyl maltoheptaoside as function of the maltotetraose concentration. Experimental data obtained with duplicate experiments (experiment 1: squares experiment 2: triangles) The solid red line describes the model data based on the kinetic scheme in Figure 6 and the dashed blue line describes the model data based on the scheme in Figure 2. Conditions: CE = 1.0∙10-2 g∙l-1, T = 40°C, pH = 6.5. Without carbohydrates ([S2] = 0) the α-amylase activity is 100% which equals 1.51 ± 0.08µmol∙mg-1∙min-1.
A more general kinetic model has been developed that was based upon the kinetic scheme in figure 6c. This model can be used to describe and explain the observed phenomena occuring in the presence of all types of carbohydrates. It has been used for the description of the α-amylase activity as a function of the glucose, sucrose, maltose, maltotriose, maltotetraose, maltopentaose, maltohexaose and maltoheptaose concentration (see figure 7). Depending on the carbohydrate type and concentration, the measured α-amylase activity can be 75 % lower than the actual α-amylase activity. The resulting kinetic model that has been developed can be used to correct for these effects in order to obtain the actual amount of active enzyme.
The Ceralpha method was used to measure the α-amylase activity during several hydrolysis experiments. Besides the α-amylase activity we also measured the carbohydrate concentration during these hydrolysis experiments. We have used maltodextrins (partly hydrolysed starch), potato starch and wheat starch as a substrate and focussed on the gelatinization, liquefaction and saccharification steps. Several process parameters, such as substrate- and enzyme concentration, and temperature were investigated.
Gelatinisation of starch in concentrated starch-water mixtures
Gelatinisation of starch is required for several industrial applications, e.g. to size textile, to coat paper, to thicken food and to make starch accessible for enzymatic hydrolysis. During gelatinisation, starch granules take up heat and water, swell, lose crystallinity and leach amylose. If the amount of water is insufficient to provide complete swelling and disruption of the starch granules, only part of the crystallinity of the granules is lost. The remaining crystallinity only disappears after heating to higher temperatures.
Native wheat starch was used for our experiments. Gelatinisation measurements were based on birefringence, differential scanning calorimetry (DSC), wide angle X-ray scattering (WAXS) and amylose-iodine complex formation were used. For the gelatinisation of 10 w/w % starch-water mixtures, a batch reactor equipped with anchor stirrer was used. Concentrated starch-water mixtures were gelatinised or melted in a compression molder.
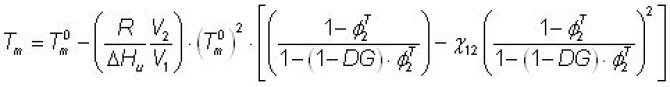
Constants: Tm0 = melting temperature of pure starch, R = gas constant, DHu = heat of fusion per repeating unit, V1, V2 = molar volumes of the diluent and the repeating unit of starch, c12 = Flory-Huggins polymer-diluent interaction parameter.

Figure 8: Gelatinisation curve of 10% w/w% wheat starch-water mixtures.
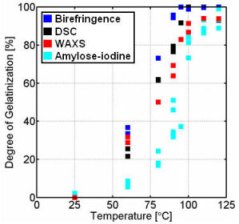
Figure 9. Gelatinisation curve of 60% w/w% wheat starch-water mixtures.
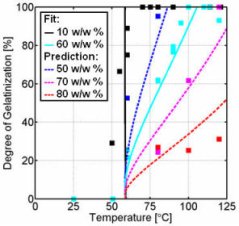
- If 10 w/w % wheat starch-water mixtures were gelatinised, each method resulted in approximately the same gelatinisation curves (Figure 8). In case the gelatinisation of a 60 w/w % wheat starch-water mixture was followed as a function of the temperature, each method resulted in a different gelatinisation curve (Figure 9). The differences can be explained by considering the phenomena that take place during the gelatinisation at excess and limiting water conditions.
- The adapted Flory equation provides a quantitative description of the observation that the required temperature for complete gelatinisation increases with an increasing starch-water ratio and it is therefore a useful tool to estimate the degree of gelatinisation as function of treatment temperature and concentration (see Figure 10).
Output
a. The effect of carbohydrates on α-amylase activity measurements. Baks, T., Janssen, A.E.M., Boom, R.M., (2006) Enzyme Microb. Technol. 39: 114-119 Abstract ; Full Text
b. A kinetic model to explain the maximum in α-amylase activity measurements in the presence of small carbohydrates. Baks T, Janssen A.E.M., Boom R.M. (2006) Biotechnology and Bioengineering 94 (3):431-440. Abstract ; Full Text (pdf)
c. Comparison of methods to determine the degree of gelatinisation for both high and low starch concentrations. Baks, T., Ngene, I.S., van Soest, J.G., Janssen, A.E.M., Boom, R.M. (2006) Carbohydrate Polymers. 67: 481-490 Abstract ; Full Text (pdf)
d. Stochastic model for predicting dextrose equivalent and saccharide composition during enzymatic starch hydrolysis. Besselink T, Baks T, Janssen AEM, Boom RM. 2007. Biotechnology and Bioengineering 100 (4): 684-697 Full Text
e. Effect of gelatinisation and hydrolysis conditions on the selectivity of starch hydrolysis with α-amylase from B. licheniformis. T. Baks, M.E. Bruins, A.M. Matser, A.E.M. Janssen, R.M. Boom. (2008) Journal of Agricultural and Food Chemistry. 56(2): 488-495. Full Text
f. The effect of pressure and temperature on the gelatinisation of starch at various starch concentrations. T. Baks, M.E. Bruins, A.E.M. Janssen, R.M. Boom. (2008) Biomacromolecules. 9(1): 296-304. Full Text
g Towards an optimal process for gelatinisation and hydrolysis of highly concentrated starch-water mixtures with α-amylase from B. licheniformis. Baks T, Kappen FHJ, Janssen AEM, Boom RM. (2008) J Cereal Sci. 47 (2): 214-225 Full Text
h Process development for enzymatic starch conversion at high concentrations. Alternatives for brewing? Baks T, Janssen AEM, Boom RM. 2007 Proceedings of 31st EBC congress, Venice, Italy.
i. Process development for gelatinisation and enzymatic hydrolysis of starch at high concentrations. Baks, T. (2007) Abstract ; Full Text
References
1. Schenck FW. Starch hydrolysates - An overview. Int Sugar J 104:82-89.
2. Klibanov AM. 1983. Stabilization of enzymes against thermal inactivation. Adv Appl Microbiol 29:1-28.
3. Krishnan T, Chandra AK. 1983. Purification and characterization of α-amylase from Bacillus licheniformis CUMC305. Appl Environ Microb 46:430-437.
4. De Cordt S, Hendrickx M, Maesmans G, Tobback P. 1994. The influence of polyalcohols and carbohydrates on the thermostability of α-amylase. Biotechnol Bioeng 43:107-114.
5. Chaplin MF, Bucke C. 1990. The large-scale use of enzymes in solution. In: Chaplin MF, Bucke C, Enzyme technology, Cambridge:Cambridge University Press. p 138-166.
6. Ceralpha: α-Amylase assay procedure (CER 07/00) Test Kit Booklet, Megazyme International Ireland Ltd., Bray, County Wicklow, Ireland.
7. McCleary BV, Sheehan H. 1987. Measurement of cereal α-amylase: a new assay procedure. J Cereal Sci 6:237-251.
8. Sheehan H, McCleary BV. 1988. A new procedure for the measurement of fungal and bacterial α-amylase. Biotechnol Tech 2:289-292.
9. McCleary BV, McNally M, Monaghan D, Mugford DC. 2002. Measurement of α-amylase activity in white wheat flour, milled malt, and microbial enzyme preparations, using the Ceralpha assay: collaborative study. J AOAC Int 85:1096-1102.
10. Hiromi K. 1970. Interpreatation of dependency of rate parameters of the degree of polymerization of substrate in enzyme-catalysed reactions. Evaluation of subsite affinities of exo-enzyme. Biochem Biophys Res Commun 40:1–6.
11. Allen JD, Thoma JA. 1976. Subsite mapping of enzymes. Application of the depolymerase computer model to two α-amylases. Biochem J 159:121-132.
12. Flory, P.J. (1953). Principles of Polymer Chemistry. Ithaca: Cornell University Press.