Forceful Predators: The Power of Immune Cells in Conquering Their Targets
WIAS Magazine - Summer edition 2024
Research Overview
Keywords: Phagocytosis, Cellular force measurements, Friction, Soft hydrogel microparticles
The immune system is essential in protecting the body from outside invaders, such as pathogens and other foreign substances. It also protects the organs inside the body by removing defective cells. The clearance process of these targets is executed by a subset of immune cells through a process called phagocytosis (1). Although mainly executed by certain specialized immune cells (professional phagocytes), the process is executed by several other cell types (non-professional phagocytes) and is essential throughout the entire organism. During phagocytosis, immune cells engulf and subsequently eliminate their targets inside the cells (Figure 1A). The significance of phagocytosis in maintaining health is underscored by the detrimental consequences arising from its malfunctioning. Defective clearance is implicated in the pathophysiology of various health conditions and diseases, such as atherosclerosis, inflammation, cancer, autoimmunity, and infectious diseases (2–6). Therefore, unraveling the principles underlying phagocytosis is of significant interest for understanding and addressing these conditions effectively.
The efficiency of phagocytosis is highly affected by the physical characteristics of the encountered target, such as size and shape. During the process, the cell generates forces to both pull and wrap around the target. Specifically, immune cells exert large constrictive forces on their targets (7,8). The extent of these forces is affected by several target properties, including stiffness, a crucial regulator for phagocytosis which can vary significantly among natural targets (9). Stiffer targets are generally eliminated more efficiently than softer ones, with immune cells exerting larger compressing forces on them (7,8,10). Interestingly, cancer cells are typically softer than surrounding tissue cells. Novel therapeutic strategies target cancer cells to induce phagocytosis for elimination. However, the low stiffness of cancer cells may hinder the effectiveness of these approaches. Thus, a deeper understanding of the generated forces during phagocytosis is essential for optimizing these therapeutic strategies.
In my project, we aim to further investigate the role of the (constrictive) forces in a novel mechanistic model for phagocytosis. We hypothesize that the observed compressive forces are a tool to generate sufficient friction, allowing immune cells to get “grip” on their target and facilitate wrapping around the target. Adequate friction facilitates forward movement, while insufficient friction leads to “slipping" around the target surface. This is analogous to walking on ice (slipping, insufficient friction) versus walking on a road (forward movement, sufficient friction). The goal is to determine how the generated friction affects the efficiency of wrapping, and thereby elimination, of different targets. Utilizing friction as a model for phagocytosis would increase the flexibility for immune cells to conquer their targets. This mechanistic model gives additional insights in several other physical cellular processes and could potentially reveal whether modulation of friction is a strategy of natural targets to evade the immune system.
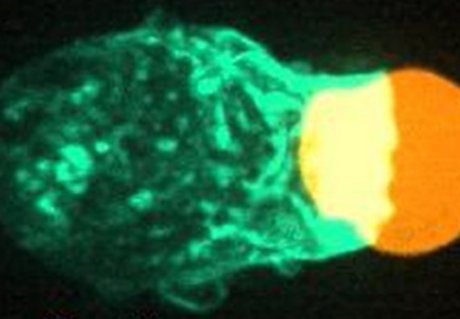
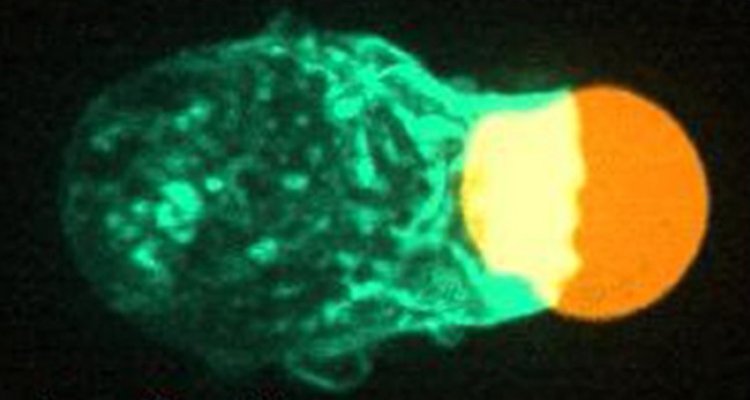
To quantitatively analyze force and friction generation during the process we combine multiple technologies. First, we utilize (rod-like) soft hydrogel particles as adaptable artificial targets for this purpose (Figure 1a). These targets are advantageous due to their uniformity, potential to perform force measurements (they work as force sensors), and ability to adjust stiffness (8). By chemically attaching specific compounds on these artificial targets, we can replicate the properties of the native pathogens (using antibodies) and defective cells (using lipids). These targets undergo phagocytosis by immune cells and through imaging with confocal microscopy combined with custom software, highly detailed data of the dynamics of phagocytosis and the involved cellular forces will be obtained (Figure 1B) (8). By systematic variation of parameters such as target shape, target stiffness and immune cell genetic perturbations, we aim to get a detailed view of how these factors affect force and friction generation and their impact on target elimination. This information aids in understanding how cellular forces can be utilized and compromised, contributing to the understanding of immune cell dysfunction related diseases and for improving therapeutic strategies.
Figure 1, Using adaptable artificial targets to measure force generation in phagocytosis. (A) An immune cell (magenta) engulfing an artificial soft hydrogel microparticle (cyan) as target. (Left) Partly internalized, (right) fully internalized. (B) (Left) Microscopy image of an immune cell interacting with a spherical artificial target. (Right) 3D reconstruction of the target with applied forces, part of the immune cell interacting with the target (white) and the part not interacting with the immune cell (pink). This methodology will be developed and applied to the rod-like particles.
- Lim JJ, Grinstein S, Roth Z. Diversity and Versatility of Phagocytosis: Roles in Innate Immunity, Tissue Remodeling, and Homeostasis. Front Cell Infect
Microbiol [Internet]. 2017 May 23. - Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nature Immunology 2015 16:9.
- Szondy Z, Garabuczi É, Joós G, Tsay GJ, Sarang Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: therapeutic implications. Front Immunol. 2014;5(AUG):354.
- Yurdagul A, Doran AC, Cai B, Fredman G, Tabas IA. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front Cardiovasc Med. 2017 Jan 8;4:86.
- Doran AC, Yurdagul A, Tabas I. Efferocytosis in health and disease. Nature Reviews Immunology 2019 20:4 [Internet]. 2019 Dec 10.
- White CJ, Gallin JI. Phagocyte defects. Clin Immunol Immunopathol [Internet]. 1986 [cited 2023 Sep 27];40(1):50–61.
- Beningo K, science YWJ of cell, 2002 undefined. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. journals.biologists.comKA Beningo, Y WangJournal of cell science, 2002•journals.biologists.com.
- Vorselen D, Wang Y, de Jesus MM, Shah PK, Footer MJ, Huse M, et al. Microparticle traction force microscopy reveals subcellular force exertion patterns in immune cell–target interactions. Nature Communications 2020 11:1.
- Vorselen D, Labitigan RLD, Theriot JA. A mechanical perspective on phagocytic cup formation. Curr Opin Cell Biol. 2020 Oct 1;66:112–22.
- Vorselen D, Barger SR, Wang Y, Cai W, Theriot JA, Gauthier NC, et al. Phagocytic “teeth” and myosin-ii “jaw” power target constriction during phagocytosis. Elife. 2021 Oct 1;10.