What is up with that gut? Using zebrafish to model host-microbe-feed interactions
WIAS Magazine - Spring edition 2023
Research Highlight
I recently finished the PhD program that I carried out in the groups of Cell Biology and Immunology, Aquaculture and Fisheries and the Laboratory of Microbiology. In this brief article I intend to summarize the research topics of my thesis, as well as encourage PhDs with a few tips that worked for me during this journey. You can find my complete PhD thesis: https://edepot.wur.nl/583665
The research project
Gut health is an umbrella term defined by an effective food digestion and absorption, the absence of gastrointestinal symptoms and disease, a balanced immune status, and a stable intestinal microbiota composition (Bischoff, 2011). Modelling all aspects about gut health simultaneously is a great challenge and during my PhD I focused on characterizing feed-derived gut inflammation by using zebrafish as a model. Zebrafish are widely used in research due to their unique properties, among which their small size, abundant offspring, full annotated genome, feasibility to generate knock-outs by CRISPR-Cas, sequential immune development, transparency in their early stages that combined with transgenesis allows live visualization of fluorescent cells in a full organism, to name a few (picture 1).
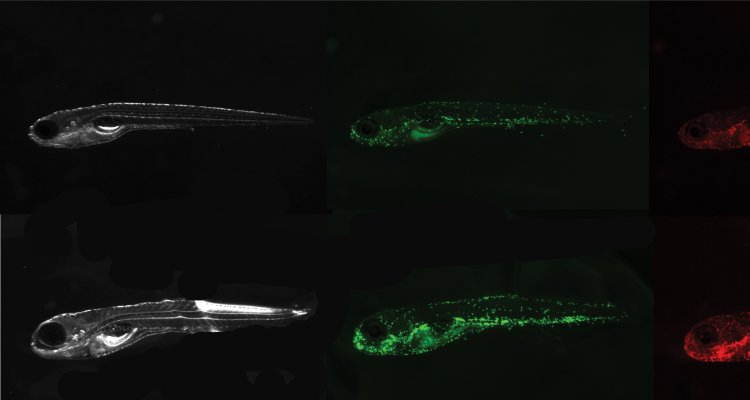
The first challenge in my PhD was to find a feed-derived substance with pro-inflammatory properties to study intestinal inflammation in zebrafish. Soybean meal (SBM) inclusion in aquafeeds promoted enteritis in several fish species (Bakke-McKellep et al., 2000; Hedrera et al., 2013; Urán, Gonçalves, et al., 2008; Urán, Schrama, et al., 2008) and soy saponin was proven to be the responsible anti-nutritional factor within SBM to induce the inflammatory response (Augustin et al., 2011; Krogdahl et al., 2015). Zebrafish larvae showed an increase in neutrophils and macrophages (innate immune cells) upon saponin exposure (picture 1). Moreover, the microbial composition of the fish exposed to saponin was significantly different than the controls, chapter 2 or (López Nadal et al., 2018). For a better understanding of the effect of the altered microbiota on the immune response we exposed zebrafish larvae to antibiotic-treated adult zebrafish gut content. Upon confirmation that zebrafish larvae were colonized depending on the microbes exposed, we challenged the zebrafish larvae with saponin. Interestingly, the immune response of the larvae depended on the microbiota composition of the individuals, chapter 3. To further characterize feed-derived inflammation, we explored other physiological fish responses to inflammation. Similarly to the previously described “behavioural-fever” upon viral or bacterial infection (Reynolds et al., 1976), fish spent more time in warm waters when inflamed (picture 2). Saponin-exposed fish showed increased cxcl8a (chemokine that recruits neutrophils) and neutrophil presence. Interestingly, cxcl8a-deficient larvae lost their thermal preference compared to controls, chapter 4.
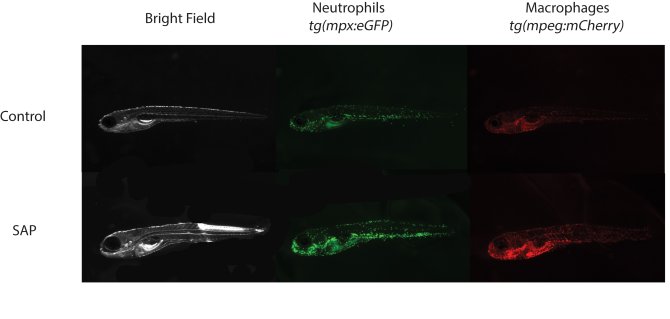
Figure 1: Zebrafish larvae control and exposed to 0.5mg/ml of soy saponin in E2 media. Representative pictures for the quantification of GFP signal (neutrophils) and mCherry signal (macrophages).
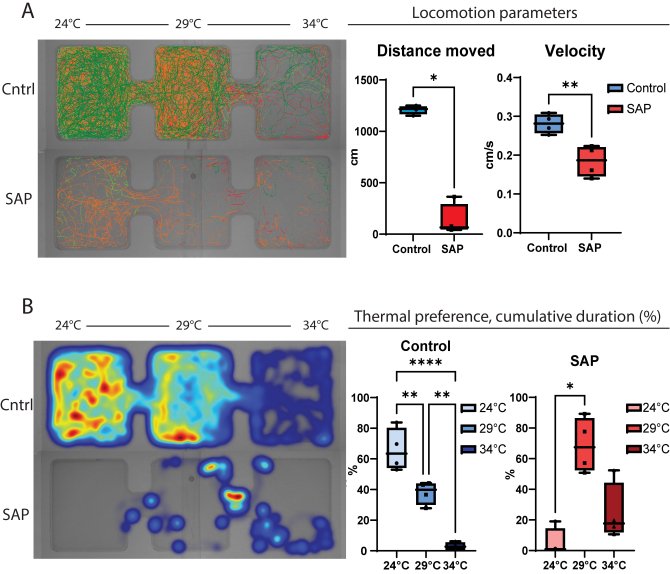
Figure 2: A) Left: Fish track visualization (n=4 per arena; top: Tg control fish, bottom: Tg saponin-exposed fish). Right: Locomotion parameters; distance moved in centimeters and velocity in centimeters/seconds. B) Left: Fish location heatmap (n=4 per arena; top: Tg control fish, bottom: Tg saponin-exposed fish) color scale blue (less time spent) to red (more time spent). Right: Individual fish cumulative duration (%) the 24°C zone, 29°C and the 34°C zone. 10 minutes acclimatization + 2h trial. Man-Whitney t-test for distance moved, un-paired t-test for velocity, one way ANOVA test with Tukey’s multiple comparison post-hoc test. *p<0.05, **p<0.005, ****p<0.0001.
To setup a comprehensive experiment to assess intestinal health, we summarized the molecular mechanisms of host-microbe-immune interactions in the zebrafish gut and we reviewed the previously published literature on the effect of feed (supplements) on the immune system in zebrafish. Many studies used only larval stages to assess the effect of feeds on zebrafish (gut) health. Moreover, the majority of the scientific claims derived from studies using one or two parameters to evaluate gut health, so we concluded that in (zebra)fish a more comprehensive view on gut health is needed to assess the properties of novel feeds (ingredients), chapter 5 or (López Nadal et al., 2020). Several high throughput datasets deriving from multiple laboratory techniques can be analysed to understand zebra(fish) gut health. We created oil-coated pellets of the golden standard growing zebrafish diet (high on fish meal), and also two other diets mimicking the same composition but with an inclusion soy saponin and of sodium butyrate. We isolated total RNA from the guts of the fish fed at the three diets and performed host transcriptomics with matched microbiota analyses as well as high-throughput quantitative histology to understand the effect of saponin and butyrate on gut health in zebrafish (picture 3). We found that saponin promoted gut inflammation (in the transcriptomics profile and the tissue samples) and microbial disturbances compared to the controls. Interestingly, butyrate-fed fish presented inflammation features comparable to the saponin-fed fish. Although there is literature reporting beneficial effects of butyrate on (zebra)fish, our comprehensive analyses point out that an exogeneous feeding of butyrate may not be beneficial for growing fish, chapter 7 or (López Nadal et al., 2023) . Despite the findings reported on my PhD there is much more to study on gut health and this is the reason why I was appointed as a post-doctorate fellow at the Host-Microbe Interactomics to understand how the immune system can control the presence of several bacteria that are potentially harmful for the zebrafish.
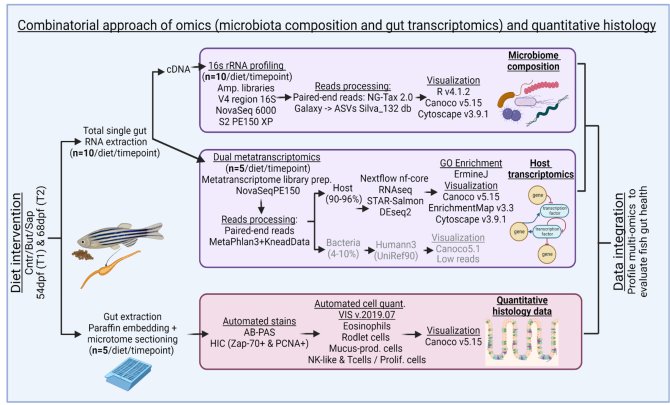
Figure 3:Combinatorial approach employed: total RNA was extracted from single zebrafish gut fed on different diets for both timepoints. Aliquots of total RNA were used for cDNA. For the 16S rRNA gene profiling, amplicon libraries of the V4 region of the 16S RNA gene were generated from the cDNA synthetized. NG-Tax 2.0 Galaxy was sued to obtain the ASVs. R v4.1.2., Canoco v5.15, and Cytoscape v3.9.1 were used for results visualization. For transcriptomics, the cDNA libraries were sent to NovaSeq 6000 PE150 for sequencing. MetaPhlAn 3.0 and KneadData were used to trim the overrepresented sequences. Nf-core/rnaseq Nextflow pipeline was used for processing of the reads with the GRCz11 genome assembly. The results were visualized by R v.4.1.2, Canoco v5.15, and ErmineJ was used for the GO Enrichment analysis. The histological samples were extracted and embedded in paraffin and sectioned using a microtome. AB-PAS and HIC stains were automated. Samples were digitally scanned and an automated quantification of the histological parameters was performed using VIS v.2019.07, Canoco v5.15, and GraphPad Prism v9.0.0 to visualize the results. The data integration was performed using heatmaps of normalized relevant parameters from all datasets, both timepoints and all diets.
The demanding challenge
The PhD is a stressful journey in which you gain knowledge about certain scientific topics and even more about yourself. How you manage your expectations, your perfectionism, your failures, your tedious laboratory routine, your endless analyses, or your supervisors, is crucial for the completion of your project. Often, it can be a lot, combined with other circumstances in your life. My small piece of advice is: reach out! Reach out to your supervisors, they may be able to help you out. Talk to your friends about what is worrying you: about your work, about your fears in the project and about your feelings. Seek for professional help if you sense you might need it; going to the therapist is investing in yourself. Get out there, practise sports and eat properly. Invest time on your hobbies, take time for yourself, and always remember: in the end a PhD is a scientific always-too-big project that if for any reason you are not able to complete for sure you will have learnt a lot for the time you worked on it.
References:
Augustin, J. M., Kuzina, V., Andersen, S. B., & Bak, S. (2011). Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry, 72(6), 435–457.
Bakke-McKellep, A. M., McL Press, C., Baeverfjord, G., Krogdahl, Å., & Landsverk, T. (2000). Changes in immune and enzyme histochemical phenotypes of cells in the intestinal mucosa of Atlantic salmon, Salmo salar L., with soybean meal-induced enteritis. Journal of Fish Diseases, 23(2), 115–127. https://doi.org/10.1046/j.1365-2761.2000.00218.x
Bischoff, S. C. (2011). “Gut health”: a new objective in medicine? BMC Medicine, 9(1), 24. https://doi.org/10.1186/1741-7015-9-24
Hedrera, M. I., Galdames, J. A., Jimenez-Reyes, M. F., Reyes, A. E., Avendaño-Herrera, R., Romero, J., & Feijóo, C. G. (2013). Soybean Meal Induces Intestinal Inflammation in Zebrafish Larvae. PLoS ONE, 8(7), 1–10. https://doi.org/10.1371/journal.pone.0069983
Krogdahl, Å., Gajardo, K., Kortner, T. M., Penn, M., Gu, M., Berge, G. M., & Bakke, A. M. (2015). Soya Saponins Induce Enteritis in Atlantic Salmon (Salmo salar L.). Journal of Agricultural and Food Chemistry, 63(15), 3887–3902. https://doi.org/10.1021/jf506242t
López Nadal, A., Boekhorst, J., Lute, C., van den Berg, F., Schorn, M. A., Bergen Eriksen, T., Peggs, D., McGurk, C., Sipkema, D., & Kleerebezem, M. (2023). Omics and imaging combinatorial approach reveals butyrate-induced inflammatory effects in the zebrafish gut. Animal Microbiome, 5(1), 15.
López Nadal, A., Ikeda-Ohtsubo, W., Sipkema, D., Peggs, D., McGurk, C., Forlenza, M., Wiegertjes, G. F., & Brugman, S. (2020). Feed, Microbiota, and Gut Immunity: Using the Zebrafish Model to Understand Fish Health . In Frontiers in Immunology (Vol. 11). https://www.frontiersin.org/article/10.3389/fimmu.2020.00114
López Nadal, A., Peggs, D., Wiegertjes, G. F., & Brugman, S. (2018). Exposure to Antibiotics Affects Saponin Immersion-Induced Immune Stimulation and Shift in Microbial Composition in Zebrafish Larvae. Frontiers in Microbiology, 9(October), 1–16. https://doi.org/10.3389/fmicb.2018.02588
Reynolds, W. W., Casterlin, M. E., & Covert, J. B. (1976). Behavioural fever in teleost fishes. Nature. https://doi.org/10.1038/259041a0
Urán, P. A., Gonçalves, A. A., Taverne-Thiele, J. J., Schrama, J. W., Verreth, J. A. J., & Rombout, J. H. W. M. (2008). Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish and Shellfish Immunology, 25(6), 751–760. https://doi.org/10.1016/j.fsi.2008.02.013
Urán, P. A., Schrama, J. W., Rombout, J., Obach, A., Jensen, L., Koppe, W., & Verreth, J. A. J. (2008). Soybean meal‐induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquaculture Nutrition, 14(4), 324–330.


