Taking the driver's seat during inflammation: manipulating Tnfα and its receptors in zebrafish with CRISPR/Cas9
WIAS Magazine - Summer edition 2022
Research Light
Key words: Inflammation, Tumour necrosis factor-alpha, zebrafish, CRISPR/Cas9, Porsche
One of the main drivers of Inflammation is Tumour necrosis factor-alpha
The engine is powering up, the heat is increasing, the wheels of inflammation are turning. In other words, the immune system is performing crucial work: I) It is ready to fight off infection and II) start wound healing. That is not all, inflammation can also be expensive as it can cause collateral damage to healthy cells and turn into chronic disease. Therefore, the complex process of inflammation, involving many different immune cells, should be well-organised by signalling molecules. One of the main drivers of inflammation is the signalling molecule Tumour necrosis factor-alpha (TNFα).
TNFα drives inflammation for better or worse depending on how much ‘gas it gives’ to the inflammatory process (figure 1). If TNFα, our main inflammation driver, gives to little gas, inflammation will be too mild, and infection will thrive. On the opposite side, if our main driver gives too much gas and goes ‘pedal to the metal’, the inflammation will be too severe, cause damage to healthy cells and possibly turn chronic. So, what we need, is TNFα to adhere to the inflammation speed limit to have all benefits, including protection against infections and
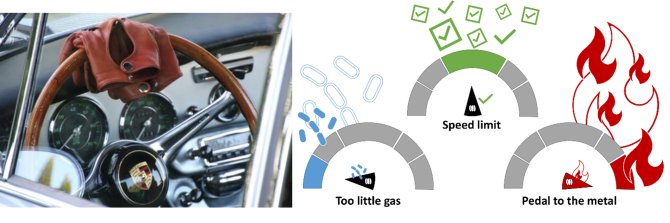
Figure 1. If you drive a Porsche, driving too slow or too fast will get you in trouble, it is best to stick to the speed limit. Similarly, TNFα, one of the main drives of inflammation, should stay within the inflammation speed limit.
We aim to understand the role of TNFα (‘our main driver’) during inflammation. In this article, we focus on how combining our animal model zebrafish (‘our Porsche’), with CRISPR/Cas9 gene-editing (‘our specialized DNA design tool’) allows us to take the driver's seat during inflammation on the road towards understanding the functioning of TNFα during inflammation.
What do zebrafish and Porsches have in common?
Understanding complex processes like inflammation, which involve many cells and molecules, can be a real challenge. A live animal model is therefore our best option since it best recapitulates the complexity of the process. To this end, we chose zebrafish, small striped fish, widely used in scientific research especially for the ease and efficiency of their gene-editing tools.
Here are the three most important things zebrafish and Porsches have in common:
- As driving a Porsche makes you feel alive, inflammation can be studied in zebrafish while they are alive. Viewed under a microscope, the transparency of young zebrafish allows you to look straight into the fish and see immune cells in action right when and where the inflammatory response happens in a living organism.
- Porsche is the only car that can be bought in any colour of your choice, just as there is almost no limit to the number of so-called zebrafish transgenic lines that can be made. These transgenic lines express colourful fluorescent proteins to mark the presence of immune cells or signalling molecules like Tnfα.
- Porsche success relies on strong ‘design DNA’, as zebrafish relies on the quality of its genome annotation, implying that the DNA sequence of all its genes is available, and can be used to design CRISPR/Cas9 complexes, strong tools to manipulate (redesign) genes.

Figure 2. The three main things Porsches & zebrafish have in common.
All combined, we have an animal model that can be observed alive, in a non-invasive way, in which we can see immune cells and identify which of these produce Tnfα depending on the fluorescence present in the transgenic line. So, we can really see which cell types are involved in the inflammatory process, how fast they are and if they are activated to produce signalling molecules such as Tnfα. Plus, we can study what happens to the cells and their behaviour when we manipulate Tnfα or it’s receptors by mutating their genes with the CRISPR/Cas9 tool.
CRISPR/Cas9: getting under the hood with the right tools
Only with the right tools will we get to understand more about how Tnfα functions. The best in our toolbox is CRISPR/Cas9: the molecular scissors. Part of the CRISPR/Cas9 complex recognises short DNA sequences through a build-in navigation system: the so-called guide-RNA (gRNA). Upon recognition by the gRNA, Cas9 (the scissor) makes a cut through both strands of DNA. When the cell tries to repair this cut, it does not always do so accurately, leaving a scar (mutations) that will effectively knock-out a gene. Put it more simply: the gene no longer functions (figure 3). Since we can design or reprogram the navigation system, we can target genes of our interest, including Tnfα and its receptors, with CRISPR/Cas9 complexes as a specialised, precise, surgical tool.
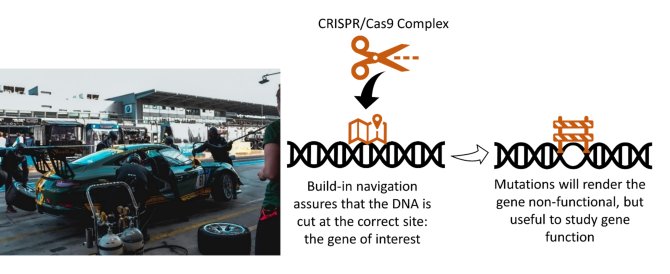
Figure 3. What goes on at a pitstop can make or break a race. CRISPR/Cas9 as gene-editing (redesign) tool can make or break a gene. When the complex recognises the correct site in the DNA and it will make a cut. This cut in the DNA in turn can lead to mutations that renders the gene non-functional, but useful for us to study our genes of interest.
Let’s get to know CRISPR/Cas9 as DNA editing (redesign) tool by targeting a pigment gene named slc24a5 as an example.
- Design: The gRNA in the CRISPR/Cas9 complex is designed to recognise a specific site of the gene Once the complex is in place it will cut the DNA leading to mutations. Before this can happen however, the complex needs to be delivered.
- Delivery: All cells of the zebrafish should be edited, meaning that all cells should have the same mutations, leading to the gene being knocked-out. To achieve this, fertilized zebrafish eggs, so young to have only one big cell, are micro-injected under the microscope with CRISPR/Cas9 solution using a tiny glass needle. This way the target gene in the one big cell will be mutated by the CRISPR/Cas9 complex, and all cells derived from that original single cell will carry the same mutation.
- Detection: Mutations in the gene, resulting from DNA editing (redesign), need to be detected. This can be done by observing how the fish looks (phenotype) and by analysing the DNA sequence. In this example, if the gene is knocked out, zebrafish will not develop their typical pigmentation and instead, will become golden. The success of knocking out the pigment can already be seen in 2-day-old zebrafish by comparing the darkness of the eyes and level of pigmentation throughout the body (figure 4). To be sure, mutations in the DNA sequence need to be further validated with molecular techniques, such as sequencing.

Figure 4. Porsches can come with or without stipes. Wild-type ‘normal’ zebrafish have stipes. When they are in the larval stage you see the pigment as black dots over the body and in the black eye (left larva). To get a larva without stipes, it is possible to edit (redesign) the pigment gene sl24a5 with CRISPR/Cas9. Mutated larvae have lost their stipes: no pigment spot over the body and transparent eyes (right larva).
The road towards understanding Tnfα
To take the driver's seat during inflammation, we designed CRISPR/Cas9 complexes to knock out Tnfα or its receptors and study how this affects inflammation caused by injury or infection. In these cases, evaluating deviations from the normal phenotype, caused by mutations in Tnfα or its receptors, is much more complex than evaluating the mutation of a pigment gene. This is because these mutations do not cause any ‘visible’ change to the colour or behaviour of the fish itself. Instead, we must pay close attention to changes at the cellular level; we have to look inside the fish to see the effects of these mutation on cell behaviour; for example, how fast they are, if and when they are activated to produce signalling molecules during inflammation. We will determine what changes occur during the complex dynamics of inflammation when Tnfα and/or its receptors are present or knocked out, all to answer fundamental questions about how Tnfα functions during inflammation.

Figure 5. Now it is time for us to get in the inflammation driver’s seat by manipulating Tnfα and its receptors with CRISPR/Cas9. This is part of our road towards understanding Tnfα functions.