It takes 22 to tango: Interleukin-22 in intestinal health and disease in zebrafish
Just like our skin, our intestinal (gut) epithelium is the barrier between us and the outside world. A proper functioning of this barrier is key to protect against unwanted translocation of pathogenic bacteria. A compromised barrier is associated with diseases in both livestock and humans, such as enteritis and inflammatory bowel disease (IBD) (1), but also obesity (2). Maintaining a healthy barrier is therefore key to disease prevention.
My PhD project will focus on the role of interleukin (IL)-22 signaling at the intestinal mucosal surface to understand its regulation in microbiota composition, (intestinal epithelial) energy metabolism and epithelial barrier function during development.
IL-22: a busy cytokine
A healthy gut epithelial barrier is the result of an intricate balance between our gut microbiota and our immune system. One of the major immune players involved in maintaining epithelial integrity is IL-22, a cytokine with broad biological functions. Its main role is at mucosal surfaces, to promote antimicrobial immunity and to support tissue repair. On top of that, IL-22 was recently implicated to play a role in metabolism, as IL-22 may increase mitochondrial metabolism and glycolysis in epithelial cells (3, 4).
In case of intestinal inflammation, IL-22 is upregulated to protect the epithelium from damage. However, IL-22 can be a double-edged sword: when its actions are uncontrolled, it may promote tumor development (5). Additionally, in colitis models it was shown that too much IL-22 may have deleterious effects. IL-10-/- mice (a model of spontaneous colitis), show an upregulation of IL-22 expression. Interestingly, it was recently shown that a double knock-out of both IL-10 and IL-22 did not lead to the development of spontaneous colitis, indicating that development of enterocolitis is in part dependent on increased IL-22 (6).
With this PhD, we hope to find out more about what determines IL-22 signaling outcome. IL-22 operates at the intersection of intestinal barrier function, immune responses and metabolic health, and we will approach IL-22 from each of these angles.
Using zebrafish to study (in vivo) IL-22 signaling
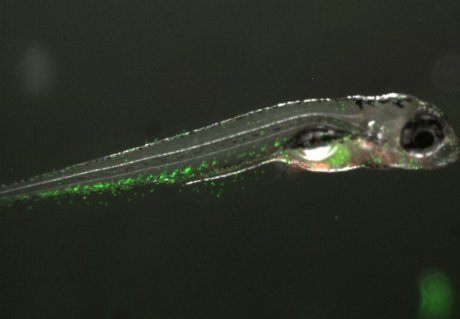
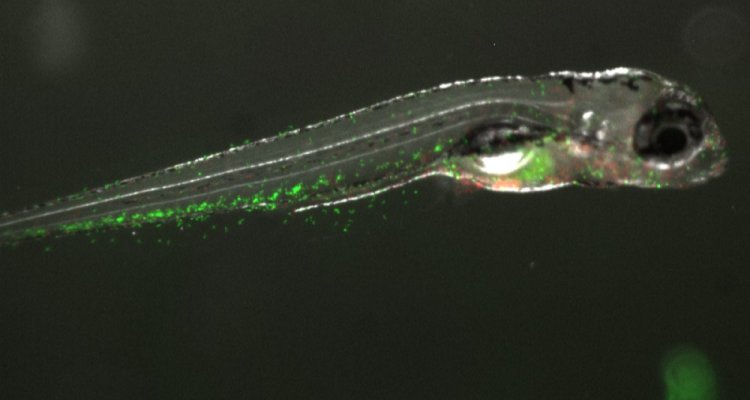
The small freshwater zebrafish (Danio rerio) is becoming more and more popular as a model organism in research. The zebrafish brings many advantages to answer our research question. One intriguing feature of these fish is that they have a sequential development of their immune system. This means that early in life, they only have their innate immunity, with their adaptive immunity starting to develop from around 10-14 days post fertilization (dpf) (7]) Coupled with the fact that zebrafish develop ex utero, this gives a unique window in which to study the innate immune system in absence of the adaptive immune system (which are both present in early life in for example mice). Another cool feature of these fish is their early-life transparency, which enables in vivo tracking of immune cells in transgenic fish that have fluorescently labelled immune cells. Taking into account these features, we plan to use CRISPR-Cas9 generated IL-22 signaling mutants to investigate the consequences of a lack of IL-22 signaling on intestinal health and susceptibility to inflammation. Next to this, we will use the Seahorse metabolic flux analyzer to investigate the consequences of a lack of IL-22 signaling on lipid and energy metabolism in zebrafish larvae in vivo.
All in all, I think a very exciting road lies ahead! Our research could improve our fundamental understanding of the role of IL-22 in intestinal health and disease, which could result in new strategies to maintain or restore the integrity of the intestinal barrier, thereby controlling or even preventing disease.
I am very excited to be able to study the fundamental mechanistics behind IL-22 signaling in this PhD. I really enjoy being able to dive deep into topics and go towards cellular and molecular levels to tease out mechanistic insights, and therefore I think this PhD is very fitting for me. But besides doing research, I am also looking forward to meeting new people in the field and sharing experiences.
References
1.Stange, E.F. and B.O. Schroeder. Microbiota and mucosal defense in IBD: an update. Expert Rev Gastroenterol Hepatol. 2019;13(10):963-976.
2.Teixeira, T.F., et al., Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32(9):637-47.
3.Chen, W., et al., IL-22-mediated renal metabolic reprogramming via PFKFB3 to treat kidney injury. Clin Transl Med. 2021;11(2):e324.
4.Chen, W., et al., Interleukin-22 drives a metabolic adaptive reprogramming to maintain mitochondrial fitness and treat liver injury. Theranostics. 2020;10(13):5879-5894.
5.Huber, S., et al., IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature.2012;491(7423):259-63.
6.Gunasekera, D.C., et al., The development of colitis in Il10(-/-) mice is dependent on IL-22. Mucosal Immunol. 2020;13(3):493-506.
7.Dee, C.T., et al., CD4-Transgenic Zebrafish Reveal Tissue-Resident Th2- and Regulatory T Cell-like Populations and Diverse Mononuclear Phagocytes. J Immunol. 2016;197(9):3520-3530.
8. Kidess, E., Kleerebezem, M., & Brugman, S. (2021). Colonizing microbes, IL-10 and IL-22: keeping the peace at the mucosal surface. Frontiers in Microbiology,