Virus neutralization tests against SARS-CoV-2 variants
Wageningen Bioveterinary Research (WBVR) offers virus neutralization tests (VNTs) against SARS-CoV-2 variants.
Since the outbreak of Severe Acute Respiratory Syndrome Corona Virus 2 (SARS‐CoV‐2), it has become extremely important to have well‐established and validated diagnostic and serological assays for this emerging virus. In particular, a specific assay to measure the antiviral activity of SARS-CoV-2–specific antibodies is urgently needed for: 1. COVID-19 serodiagnosis, 2. convalescent plasma therapy and 3. vaccine development.
Determining antibody efficacy
Several test platforms are available for serological testing and determining immune protection for SARS-CoV-2, including ELISA, lateral flow immunoassay, microsphere immunoassay and pseudovirus systems. These assays measure the binding of antibodies to the SARS-CoV-2 spike protein.
However, since not all spike-binding antibodies can block viral infection, these platforms do not measure antibody inhibition of SARS-CoV-2 infection. This requires a biological assay, namely the virus neutralization test (VNT). This is a highly sensitive and specific serological assay to detect the presence and magnitude of functional antibodies that prevent infectivity of a virus. The VNT is the gold standard for determining antibody efficacy.
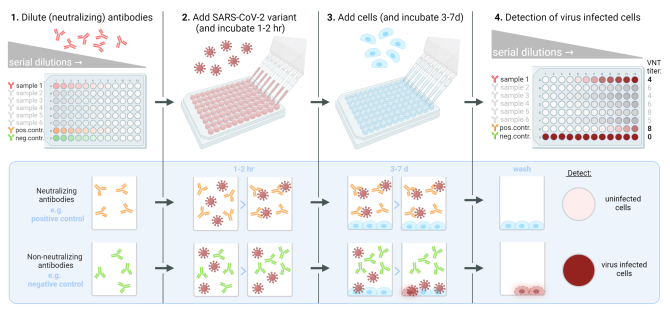
The virus neutralization test (VNT)
The performance of the SARS-CoV-2 VNT requires specialised personnel and a high containment laboratory (Biosafety Level 3). In this test, a defined quantity of a variant of SARS-CoV-2 is mixed with serial dilutions of the antibody sample to be tested; serum, supernatant, or a (monoclonal) antibody. After optimal incubation of these virus-antibody mixtures, susceptible cells are added. These cells are incubated at a temperature suitable for viral growth for 3-7 days and are then examined for the production of virus-induced cytopathic effect (CPE) or other indicators of viral growth.