Why is early life feeding important in piglets?
WIAS magazine - fall edition 2021
Research Highlight
Keywords: early life, development, fibre, microbiota, transcriptomics, pig, weaning
Early-life bacterial colonisation can be of particular importance to the overall growth and health of an animal, especially influencing intestinal and immune system development with long-term implications. This is especially relevant in pig production where post-weaning enteric infection is one of the major concerns related to the (gut) health of pigs, and is associated with economic losses and welfare problems. Commercial pig production systems involves early and abrupt weaning, which contrasts with the gradual transition from mother’s milk to solid feed in nature. Due to such abrupt weaning, a piglet is challenged with multiple stressors (including environmental-, nutritional- and psychological-) which is usually associated with changes in gut microbiota and a high incidence of diarrhoea. Therefore, we aimed to evaluate the impact of early-life feeding (pre-weaning provision of fibrous feed) on the intestinal microbiota and mucosa development in neonatal piglets, thereby preparing piglets for weaning transition, as described in the PhD thesis (1).
From two days of age, early-fed (EF) piglets were provided with a customised fibrous feed (including galacto-oligosaccharides, inulin, and resistant starch) till weaning (at 28 days of age) in addition to sow’s milk, whereas the control (CON) piglets exclusively suckled mother’s milk. Piglets were followed from birth till 2-3 weeks after weaning (day2, 7, 15, 21, 28, +2, +5, +14), where samples (rectal swabs and/or intestinal contents) were collected at pre- and post-weaning time-points to investigate gut microbiota colonisation by 16S metataxonomic analysis. Furthermore, intestinal tissue samples were collected from a subset of piglets to investigate host transcriptome analysis (RNA sequencing) as well as morphometric (histological) parameters.
The longitudinal study (2) displayed a dynamic intestinal microbiota colonisation pattern during the pre-weaning period in both treatment groups, which rapidly stabilized 2-3 weeks post-weaning. The homogenous post-weaning microbiota (day+14; Figure1) was characterised by microbial groups including Prevotella, Roseburia, Faecalibacterium, Ruminococcus, Megasphaera, Catenibacterium, Subdoligranulum, that are mostly fibre-degraders and/or SCFA producers. Remarkably, early feeding of neonatal piglets resulted in ‘accelerated maturation’ of the gut microbiota, compared to the CON piglets. The accelerated maturation was characterised by the increased rate of microbial diversity, simultaneous with the emergence of typical post-weaning-associated microbial groups (mentioned earlier; Figure 1 illustrating the proximity of EF piglets to post-weaning time-point day+14) and a more rapid decline of typical pre-weaning microbial groups (e.g., Fusobacterium, Finegoldia, Bacteroides, Eschechichia-Shigella). Moreover, we found a quantitative association between eating behaviour of EF piglets (video scores) and their microbiota signature, indicating that the piglets who spent more time at the feeding trough had a higher abundance of ‘accelerated’ microbial groups (2). In addition, early feeding increased microbial fermentation products (SCFA) in the colon and modulated intestinal development (i.e., increased weights and lengths of several intestinal tract segments; decreased jejunal villus-crypt ratio; increased abundance of proliferative cells in colon mucosa) (3). On investigating the mucosa transcriptome response, we observed maximum impact of early feeding at weaning compared to other time-points, which was followed by convergence of the transcriptome three weeks post-weaning (day+21). Similar to the microbiota finding, the host transcriptome maturation also seemed to be accelerated at weaning in EF piglets, and was more similar to the ‘homogenous/mature’ post-weaning transcriptome, compared to the CON piglets (Figure2 displaying the closer proximity of EF group to the convergent post-weaning time-point day+21). Furthermore, three days after weaning (period where pigs experience acute weaning stress), the EF piglets displayed a stronger mucosal responsiveness, reflected in the increased transcription of genes related to “immune activation”, “epithelial migration” and “wound-repair" processes necessary to maintain gut barrier integrity (during weaning transition) and jejunal morphometry (1). Notably, we also observed early-life feeding affecting animal growth and performance, illustrated by a smoother relative weight gain, tendency of a higher relative weight gain as well as reduced diarrhoea scores during the “acute phase” post-weaning, i.e., during the first week post-weaning when piglets experience maximum weaning stress. These acceleration effects are especially remarkable when we realise that the (fibrous) feed intake in suckling piglets was only a small fraction of the overall food intake, because the sow’s milk constitutes the main nutrient and energy source of the young piglet. Overall, these findings underline the importance of utilising the early-life “window of opportunity” to accelerate the intestinal development towards a relatively matured state, thereby better preparing the piglets for the post-weaning adaptation. This knowledge can lead to sustainable husbandry regimes that mimic natural conditions for the production of healthier and more resilient pigs.
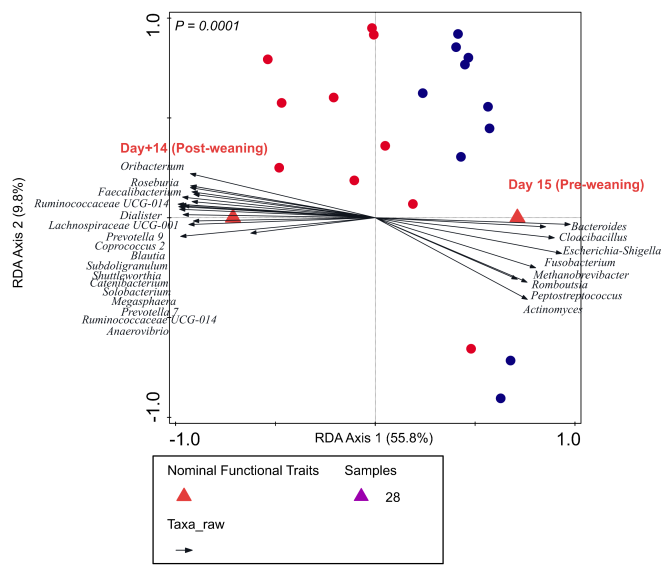
Figure 1: Acceleration of microbiota: Redundancy analysis of pre- (day15) and post-weaning (day+14) time-points along with microbial groups associated at those two time-points (P = 0.0001). Day28 samples (time-point just before weaning) that were projected as ‘supplementary’ in this plot clearly illustrates the proximity of the early-fed (EF; red circles) piglets to the post-weaning microbiota, compared to the control (CON; blue circles) piglets.
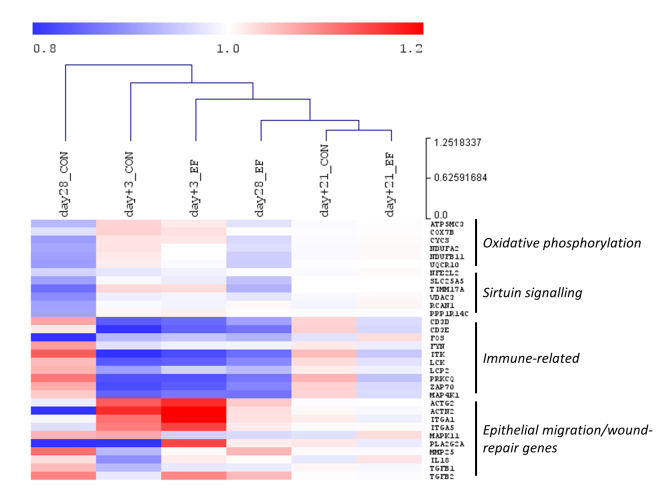
Figure 2: Acceleration of transcriptome: Unsupervised hierarchical clustering of colonic gene expression over time (d28: just before weaning; day+3: three days post-weaning; day+21: three weeks post-weaning). Representative genes from differentially expressed pathways were shown in this figure. The normalised expression values (averaged per group per time-point) are scaled by the mean value of total day+21 expression (irrespective of treatment).
1. Choudhury R. Early-life feeding in piglets: the impact on intestinal microbiota and mucosal development (PhD thesis). Wageningen University and Research; 2021.
2. Choudhury R, Middelkoop A, Gerrits WJJ, Kemp B, Bolhuis JE, Kleerebezem M. Early-life feeding accelerates gut microbiome maturation in piglets. Submitt Publ (bioRxiv 20200930320275) [Internet]. Available from: https://doi.org/10.1101/2020.09.30.320275
3. Choudhury R, Middelkoop A, Souza JG de, Veen L. van, Gerrits WJ., Kemp B, et al. Impact of early-life feeding on local intestinal microbiota and digestive system development in piglets. Sci Rep. 2021;11(4213):1–16.