Weed control
There is a subterranean war occurring in Africa with a parasite gnawing at the very roots of the existence of countless farmers. Striga – also known as witchweed – may look like a pretty flower, but wherever it casts its spell harvests are lost.
Harro Bouwmeester, Professor of Plant Physiology has been investigating the cause of the striga plague for many years. Some 40 percent of all agricultural land in Africa is infected with the seed of this parasitic weed today, resulting in huge losses in the production of sorghum, millet, maize and upland rice. The total damage is estimated to be between 7 and 13 billion dollars, while striga also threatens the food supply of around 300 million Africans.
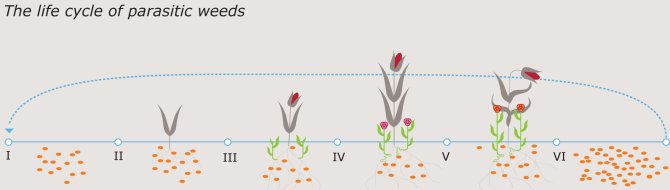
Controlling the weed is difficult. Seeds lay in the soil for long periods waiting to attach to crop roots, during which time the plague remains invisible. Although the seed does not start germinating until a crop is nearby, the striga is a very patient parasite that can even strike after years of inactivity.
Parasitic witchweed threatens food crops
The advance of striga is occurring at the same pace as the depletion of the soil. Many African fields are used for cultivation year after year without being left fallow and often without the addition of artificial fertiliser. Striga is in its element in such conditions, which is the easy explanation for the parasite’s success. Interestingly, however, it seems that the host also causes its own demise, as Bouwmeester discovered while focusing on the so-called strigolactones; the signal substance that the crops exude into the soil via their roots. These signal substances betray crops like sorghum to the striga seed. The relation between the amount of strigolactones produced by a crop and the size of the plague is clear: More strigolactones equals more witchweed.
Masochistically
So what moves the host plant to behave so masochistically? For a long time, Bouwmeester and his colleagues did not understand the evolutionary logic behind it. Until a new fan of the strigolactone signals was found in 2005: arbuscular mycorrhizal fungi that settle in the root system of the host just like the parasite. Unlike striga, however, this symbiotic fungus is beneficial to the host. In return for photosynthetic sucrose, the fungus uses its deep and widely branched fibres to extract nutrients from the soil that the host cannot reach itself. The fungus is especially skilful in extracting phosphate. When a host has a phosphate shortage, it increases its strigolactone production to attract the fungus. In the African soil, however, this leads to a disastrous version of a Trojan horse – in addition to the beneficial fungus, striga seeds also respond to the SOS, resulting in an unequal battle with calamitous consequences for the host.
A primitive way to control a striga plague is to weed all the stems of the striga. Although not a cure-all, this can save part of the harvest. African farmers who see the parasite blossom, however, know where they stand and leave the field behind in the knowledge that the harvest is lost. Unfortunately, this only worsens the striga. The blossoming parasite produces many thousands of new seeds that patiently wait in the soil for the strigolactones to tell them where to go to benefit from a new host.
Bouwmeester had previously shown that the strigo-lactones in the plant are produced from carotenes. A logical next question was how one can reduce the production of carotenes without affecting the growth of the plant. There are herbicides available that inhibit carotene production and therefore limit the production of strigolactones. This results in a 60 to 75 percent reduction in the germination of striga seeds. If African farmers would use these herbicides on their crops in very small concentrations, it would be an easy wayto combat the parasite. However, herbicides are not a perfect solution and are often unattainable or unaffordable for the majority of Africans.

Phosphate bath
A more expensive and even more unattainable option so far in many parts of Africa is adding the required phosphate to the soil via artificial fertiliser. The fertiliser solution cuts both ways: It increases yield (which is only ten percent of the maximum possible production in some places, even without striga) and eliminates the parasite by stopping the production of strigolactones. In the research some success was achieved with a more precise application of phosphate, such as giving the seeds a phosphate bath prior to sowing or putting phosphate in the soil near planted seeds. Another method is to provide seeds with a mycorrhizal fungus coating to ensure a good start.
An encouraging aspect of Bouwmeester’s research is his finding that not all varieties of rice and sorghum are equally sensitive to striga. The signal substances also play a key role in the process as he demonstrated by studying the strigolactone production of collections of rice and sorghum varieties. An important aspect here was replacing the classic bio-assays for determining the strigolactones with a quantitative analysis of the signal substances using sensitive analysis equipment (LC-MS), bought using finance from the Netherlands Organisation for Scientific Research (NWO). Of the many rice varieties studied, several produced very low amounts of signal substances and showed considerable striga resistance. Plants with many tillers produce few strigolactones and are less affected by the parasite so breeders can use this to develop resistant varieties. Cooperation with World Food Prize winner Gebisa Ejeta broughtto light sorghum varieties that also produce strigo-lactones while being far less attractive to striga. According to Bouwmeester this proves that the signal substances can change their tune. "
Striga hermonthica emergence in some rice cultivars
Striga hermonthica emergence in some representative low- and high tillering rice cultivars as determined 12 weeks after sowing. The average number ofS. hermonthica shoots emerged (between brackets) were: IAC 165 (34),IAC 1246 (29) (both lowtillering), Agee (1), Anakila (1), TN 1 (4) and Super Basmati (4) (high tillering).
Source: Genetic variation in strigolactone production and tillering in rice and its effect
on Striga hermonthica infection Muhammad Jamil et.al. Planta (2012) 235:473–484





