
Equipment
Time correlated single photon counting
Cuvet based TCSPC is used for time-resolved fluorescence measurements. It can quantify the amount of FRET, or resolve the ultrafast events in photosynthesis. It covers the range from tryptophan (UV) to chlorophyll (NIR).
The fluorescence of a sample can be monitored as a function of time after excitation by a flash of light. We use the Time Correlated Single Photon Counting (TCSPC) method to record the fluorescence time trace. Processes in the excited state(s) of fluorophores can be followed: e.g. energy transfer, solvent relaxation, etc. The rates of these processes can be determined from the lifetimes of the fluorescent intermediate and final states. Using our TCSPC set-up, lifetimes between 20 ps up to 20 ns can be measured, and it covers the wavelength range from tryptophan (UV) to chlorophyll (NIR).
Furthermore, the set-up is equipped with polarizers for time-resolved fluorescence anisotropy measurements. During the fluorescence lifetime, the changes in the orientation of the molecules can be probed. Time-resolved fluorescence anisotropy is used to obtain information about molecular rotation and homo-FRET between proteins. The rotational dynamics are determined by e.g. the shape of the protein and its aggregation state, or the viscosity of the medium surrounding the fluorescent molecule.
Typical applications: photosynthesis research, solvent environment probing, protein conformation and inter/intra-molecular distance measurements (FRET).
Background:
After absorption of light, a molecule is in one of its excited states. It can now undergo vibrational relaxation, internal conversion, solvent relaxation, energy transfer and/or fluorescence. All processes have their own rate (in s-1), and consequently all intermediate states have the own lifetime. When such an intermediate state can fluoresce, the lifetime of that state can measured. By recording the time-resolved fluorescence decay at the emission wavelength of these states (for example the blue, green and red in the figure), the lifetime of that state can be determined. The rate of the population of the state (ingrowth) and depopulation (decay) can be calculated from the traces.
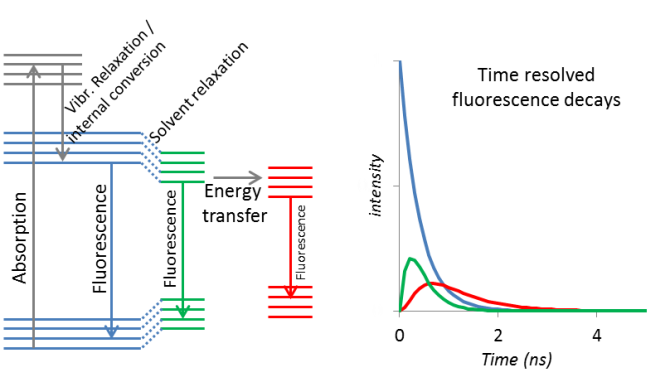
The fluorescence decay is measured by time-correlated single photon counting (TCSPC). In TCSPC, the time differences between the detection of the individual photons (fluorescence) and the subsequent next laser pulses are recorded. All these time differences are plotted in a histogram. The number of detected counts per second is kept much lower than the number of excitation pulses (1:100). A second photon in the same cycle cannot be processed and neglecting that photon will lead to (pile-up) distortion.
Equipment:
For excitation a Ti:sapphire laser is used in combination with a pulse picker and frequency converters (OPO or harmonic generator) to create 4 MHz repetition rate pulses with variable wavelength. For detection, a multichannel plate photomultiplier tube (MCP-PMT) is used.
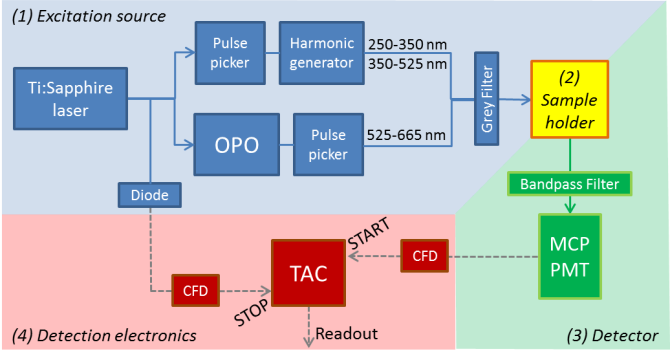
Key specifications:
Available excitation wavelengths: 250-665 nm
Available interference / cut-of filters: ranging from 300 to 850 nm
Repetition rate: 4 MHz
Instrument response: 45 ps FWHM
Can measure lifetime from 20 ps up to 20 ns
Publications
- Tian, L., Farooq, S. & Amerongen, H. van (2013). Probing the picosecond kinetics of the photosystem II core complex in vivo. Physical Chemistry Chemical Physics, 15(9), 3146-3154.
- Wientjes, E., Amerongen, H. van & Croce, R. (2013). LHCII is an antenna of both photosystems after long-term acclimation. Biochimica et Biophysica Acta. B, Bioenergetics, 1827(3), 420-426.
- Lindhoud, S., Westphal, A.H., Borst, J.W., Visser, A.J.W.G. & Mierlo, C.P.M. van (2012). Fluorescence of Alexa Fluor dye tracks protein folding. PLoS One, 7(10).
- Caffarri, S., Broess, K., Croce, R. & Amerongen, H. van (2011). Excitation energy transfer and trapping in higher plant photosystem II complexes with different antenna sizes. Biophysical Journal, 100(9), 2094-2103.
- Laptenok, S., Visser, N.V., Ruchira, A., Westphal, A., Hoek, A. van, Mierlo, C.P.M. van, Stokkum, I.H.M. van, Amerongen, H. van & Visser, A.J.W.G. (2011). A general approach for detecting folding intermediates from staedy-state and time-resolved fluorescence of single-tryptophan-containing proteins. Biochemistry, 50(17), 3441-3450.
- Lee, J.S., Koehorst, R.B.M., Amerongen, H. van & Feijen, J. (2011). Time-resolved fluorescence and fluorescence anisotropy of fluorescein-labeled poly(N-isopropylacrylamide) incorporated in polymersomes. The Journal of Physical Chemistry Part B: Condensed Matter, Materials, Surfaces, Interfaces & Biophysical, 115(45), 13162-13167.
- Malikova, N.P., Visser, N.V., Hoek, A. van, Skakun, V.V., Vysotski, E.S., Lee, J. & Visser, A.J.W.G. (2011). Green-Fluorescent Protein from the Bioluminescent Jellyfish Clytia gregaria Is an Obligate Dimer and Does Not Form a Stable Complex with the Ca2+-Discharged Photoprotein Clytin. Biochemistry, 50(20), 4232-4241.
- Visser, A.J.W.G., Vysotski, E.S. & Lee, J. (2011). Critical Transfer Distance Determination Between FRET Pairs. Photobiological Sciences Online.
- Visser, A.J.W.G. & Rolinski, O.J. (2010). Basic Photophysics. Photobiological Sciences Online.
- Borst, J.W., Willemse, M., Slijkhuis, R., Krogt, G. van der, Laptenok, S., Jalink, K., Wieringa, B. & Fransen, J.A.M. (2010). ATP Changes the Fluorescence Lifetime of Cyan Fluorescent protein via an Interaction with His148. PLoS One, 5(11), e13862.
- Haferkamp, S., Haase, W., Pascal, A.A., Amerongen, H. van & Kirchhoff, H. (2010). Efficient light-harvesting by photosystem II requires an optimized protein packing density in grana thylakoids. Journal of Biological Chemistry, 285(22), 17020-17028.
- Oort, B.F. van, Alberts, M., Bianchi, S. de, Dall'Osto, L., Bassi, R., Trinkunas, G., Croce, R. & Amerongen, H. van (2010). Effect of Antenna-Deletion in photosystem II on Excitation Energy Transfer in Arabidopsis thaliana. Biophysical Journal, 98(5), 922-931.
- Passarini, F., Wientjes, E., Amerongen, H. van & Croce, R. (2010). Photosystem I light-harvesting complex Lhca4 adopts multiple conformations: red forms and excited-state quenching are mutually exclusive. Biochimica et Biophysica Acta. B, Bioenergetics, 1797(4), 501-508.
- Visser, A.J.W.G., Laptenok, S., Visser, N.V., Hoek, A. van, Birch, D.J.S., Brochon, J.C. & Borst, J.W. (2010). Time-resolved FRET fluorescence spectroscopy of visible fluorescent protein pairs. European Biophysics Journal, 39(2), 241-253.
- Broess, K., Borst, J.W. & Amerongen, H. van (2009). Applying two-photon excitation fluorescence lifetime imaging microscopy to study photosynthesis in plant leaves. Photosynthesis Research, 100(2), 89-96.
- Visser, N.V., Westphal, A.H., Nabuurs, S.M., Hoek, A. van, Mierlo, C.P.M. van, Visser, A.J.W.G., Broos, J. & Amerongen, H. van (2009). 5-Fluorotryptophan as dual probe for ground-state heterogeneity and excited-state dynamics in apoflavodoxin. FEBS Letters, 583(17), 2785-2788.
- Broess, K., Trinkunas, G., Hoek, A. van, Croce, R. & Amerongen, H. van (2008). Determination of the excitation migration time in Photosystem II consequences for the membrane organization and charge separation parameters. Biochimica et Biophysica Acta. B, Bioenergetics, 1777(5), 404-409.
- Eremeeva, E., Markova, S.V., Frank, L.A., Lee, J., Berkel, W.J.H. van, Visser, A.J.W.G. & Vysotski, E.S. (2008). The kinetics of coelenterazine binding with apo-obelin and apo-aequorin. Luminescence, 23(2), 66-67.
- Visser, N.V., Westphal, A.H., Hoek, A. van, Mierlo, C.P.M. van, Visser, A.J.W.G. & Amerongen, H. van (2008). Tryptophan-Tryptophan energy migration as a tool to follow apoflavodoxin folding. Biophysical Journal, 95, 2462-2469.
- Almeida, R.F.M. de, Borst, J.W., Federov, A., Prieto, M. & Visser, A.J.W.G. (2007). Complexity of Lipid Domains and Rafts in Giant Unilamellar Vesicles Revealed by Combining Imaging and Microscopic and Macroscopic Time-Resolved Fluorescence. Biophysical Journal, 93, 539-553.
- Berg, P.A.W. van den, Grever, K., Hoek, A. van, Berkel, W.J.H. van & Visser, A.J.W.G. (2007). Time-resolved fluorescence analysis of the mobile flavin cofactor in p-hydroxybenzoate hydroxylase. Journal of Chemical Sciences, 119(2), 123-133.
- Nazarov, P.V., Koehorst, R.B.M., Vos, W.L., Apanasovich, V.V. & Hemminga, M.A. (2007). FRET study of membrane proteins: determination of the tilt and orientation of the N-terminal domain of M13 major coat protein. Biophysical Journal, 92(4), 1296-1305.
- Oort, B.F. van, Hoek, A. van, Ruban, A.V. & Amerongen, H. van (2007). Aggregation of ligh-harvestin complex II leads to formation of efficient excitation energy traps in monomeric and trimeric complexes. FEBS Letters, 581(18), 3528-3532.
- Oort, B.F. van, Hoek, A. van, Ruban, A.V. & Amerongen, H. van (2007). Equilibrium between quenched and nonquenched conformations of the major plant light-harvesting complex studied with high-pressure time-resolved fluorescence. The Journal of Physical Chemistry Part B: Condensed Matter, Materials, Surfaces, Interfaces & Biophysical, 111(26), 7631-7637.
- Broess, K., Trinkunas, G., Weij-de Wit, C.D. van der, Dekker, J.P., Hoek, A. van & Amerongen, H. van (2006). Excitation energy transfer and charge separation in photosystem II membranes revisited. Biophysical Journal, 91(10), 3776-3786.
- Lavalette, D., Hink, M.A., Torbez, M., Tetreau, C. & Visser, A.J.W.G. (2006). Proteins as micro viscosimeters: Brownian motion revisited. European Biophysics Journal, 35(6), 517-522.
- Somsen, O.J.G., Trinkunas, G., Keijzer, M.N. de, Hoek, A. van & Amerongen, H. van (2006). Local diffusive dynamics in DNA: A time-resolved fluorescence and molecular-dynamics study of dinucleotides with 2-aminopurine. Journal of Luminescence, 119-120, 100-104.
- Westphal, A.H., Matorin, A., Hink, M.A., Borst, J.W., Berkel, W.J.H. van & Visser, A.J.W.G. (2006). Real-time Enzyme Dynamics Illustrated with Fluorescence Spectroscopy of p-Hydroxybenzoate Hydroxylase. Journal of Biological Chemistry, 281(16), 11074-11081.
- Borst, J.W., Hink, M.A., Hoek, A. van & Visser, A.J.W.G. (2005). Effects of refractive index and viscosity on fluorescence and anisotropy decays of enhanced cyan and yellow fluorescent proteins. Journal of Fluorescence, 15(2), 153-160.
- Somsen, O.J.G., Hoek, A. van & Amerongen, H. van (2005). Fluorescence quenching of 2-Aminopurine in Dinucleotides. Chemical Physics Letters, 402(1-3), 61-65.
- Somsen, O.J.G., Keukens, L., Keijzer, M.N. de, Hoek, A. van & Amerongen, H. van (2005). Structural heterogeneity in DNA : Temperature dependence of 2-aminopurine fluorescence in dinucleotides. ChemPhysChem, 6(8), 1622-1627.
- Visser, N.V., Borst, J.W., Hink, M.A., Hoek, A. van & Visser, A.J.W.G. (2005). Direct observation of resonance tryptophan-to-chromophore energy transfer in visible fluorescent proteins. Biophysical Chemistry, 116(3), 207-212.
- Berg, P.A.W. van den, Hoek, A. van & Visser, A.J.W.G. (2004). Evidence for a novel mechanism of time-resolved flavin fluorescence depolarization in glutathione reductase. Biophysical Journal, 87(4), 2577-2586.
- Chosrowjan, H., Taniguchi, S., Mataga, N., Tanaka, F. & Visser, A.J.W.G. (2003). The stacked flavin adenine dinucleotide conformation in water is fluorescent on picosecond timescale. Chemical Physics Letters, 378, 354-358.
- Hink, M.A., Visser, N.V., Borst, J.W., Hoek, A. van & Visser, A.J.W.G. (2003). Practical use of corrected fluorescence excitation and emission spectra of fluorescent proteins in Förster Resonance Energy Transfer (FRET) studies. Journal of Fluorescence, 13, 185-188.
- Verkhusha, V.V., Pozhitov, A.E., Smirnov, S.A., Borst, J.W., Hoek, A. van, Klyachko, N.L., Levashov, V. & Visser, A.J.W.G. (2003). Effect of high pressure and reversed micelles on the fluorescent proteins. Biochimica et Biophysica Acta. General subjects, 1622, 192-195.