Project
Strain improvement of microalgae for the production of omega-3 fatty acids and fucoxanthin
Pigments and polyunsaturated fatty acids (PUFAs) are metabolites with bioactive properties for human and animal health. These macromolecules are naturally accumulated in certain species of microalgae such as Isochrysis galbana and Nannochloropsis oceanica. In the last years, interest in using microalgae as a cell factory has increased due to their fast growth rates compared to plants and the possibility of growing them in marine water and non-arable land. However, the high production and biorefinery costs only allows the success of microalgae-derived products in the high-value market. In the European MAGNIFICENT project, we aim at twisting this situation by optimizing the different steps in the process chain while developing sustainable alternatives to the current fish oil (Hasan and Halwart 2009; Olsen and Hasan 2012) or synthetic pigments (Kobylewski and Jacobson 2010). Biomass cultivation can be optimised by either targeting the process conditions or the cellular metabolism.
For a long time, the environmental conditions have been used to trigger the adaptive response of microalgae to increase the neutral lipids and/or the pigment cell content (Breuer et al. 2012; Mulders et al. 2014). However, this approach usually implies a decrease in biomass productivity. As an alternative, the cellular metabolism in microalgae still remains relatively unexplored and few robust industrial strains have been developed. Within this research, strain improvement with non-recombinant techniques will be carried out to increase the cellular content of fucoxanthin (Fx) and omega-3 fatty acids (ω-3 FA) in I. galbana and N. oceanica, respectively.
Aim
We aim at developing high-throughput methodologies for the generation, identification and isolation of fucoxanthin and omega-3 fatty acids microalgal overproducers and characterising them at laboratory and pilot scale.
Approach
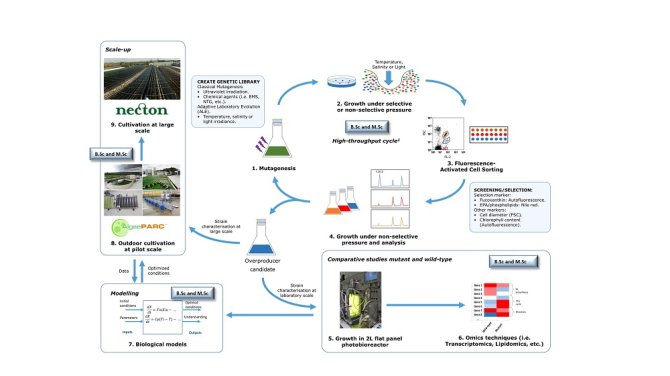
Figure 1 shows an overview of our approach. First of all, classical mutagenesis techniques are used to develop a library of mutants. Subsequently, the mutants of interest are grown under non-selective (and selective) pressure for their physiological recovery. Secondly, the mutants with the phenotype of interest will be screened and sorted with Fluorescence Activated Cell Sorting (FACS). FACS allows the separation of single cells according to their size, morphology or fluorescence derived from a fluorophore. Whereas this technique has been widely used in microorganisms like yeast or bacteria, it has been barely employed in the isolation of microalgae-overproducing strains (Hyka et al. 2013). For the screening, the fluorescence intensity of the pigments or a fluorescent probe will be used as a marker. The phenotype of the overproducer strains will be characterised and compared to the wild-type strain by using omics techniques (i.e. lipidomics, transcriptomics, etc.). Finally, we will carry out pilot studies to evaluate and improve the performance of the mutant strains before their application at industrial scale.
Thesis project
There are always plenty of possibilities for BSc and MSc thesis projects (Figure 1). Please feel free to contact and ask for details about the status of the project.
Acknowledgments
This research is part of the MAGNIFICENT project, funded by the Bio Based Industries Joint Undertaking under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 745754).
References
- Breuer, Guido et al. 2012. “The Impact of Nitrogen Starvation on the Dynamics of Triacylglycerol Accumulation in Nine Microalgae Strains.” Bioresource Technology 124: 217–26. http://dx.doi.org/10.1016/j.biortech.2012.08.003.
- Hasan, Mr, and M Halwart. 2009. “Fish as Feed Inputs for Aquaculture: Practices, Sustainability and Implications.” Practices, sustainability and implications (518): 407. http://docs.niwa.co.nz/library/public/FAOfatp518.pdf.
- Hyka, P. et al. 2013. “Flow Cytometry for the Development of Biotechnological Processes with Microalgae.” Biotechnology Advances 31(1): 2–16.
- Kobylewski, Sarah, and Michael F. Jacobson. 2010. 30 Decision Sciences Food Dyes: A Rainbow of Risks.
- Mulders, Kim J M, Packo P. Lamers, Dirk E. Martens, and René H. Wijffels. 2014. “Phototrophic Pigment Production with Microalgae: Biological Constraints and Opportunities.” Journal of Phycology 50(2): 229–42.
- Olsen, Ragnar L., and Mohammad R. Hasan. 2012. “A Limited Supply of Fishmeal: Impact on Future Increases in Global Aquaculture Production.” Trends in Food Science and Technology 27(2): 120–28. http://dx.doi.org/10.1016/j.tifs.2012.06.003.