Project
The Selfish Tumour: Loss of muscle mass in cancer patients
The loss of muscle mass and function during ageing or chronic diseases like cancer can have huge consequences for daily life. Both forms of muscle loss share similar mechanisms, but also differ to some extent. For muscle loss during ageing, a high protein diet combined with exercise can partly reverse the process. However, there is no remedy yet to treat muscle loss during (chronic) disease and specifically for muscle loss related to cancer.
A tumour can affect the whole body physiology and therefore also the muscle, even when the tumour is not located in the muscle tissue. Up to 50% of the cancer patients suffer from muscle wasting, reducing the quality of life of many patients. Moreover, it is responsible for approximately 20% of deaths in cancer patients. Therefore, it is important that we find tools to fight the consequences of this selfish tumour.
Although prevention or intervention strategies for cancer-induced muscle wasting are gaining more attention, current strategies have not met their expectations. Little attention is given so far to the effect of the tumour on the muscle micro-environment and energy status of the muscle, both of which are involved in muscle regeneration and mitochondrial physiology. In addition the tumour also promotes the immune system to produce specific cytokines. This results in chronic inflammation and inflammation of the muscle micro-environment.
We aim to elucidate the role of the muscle micro-environment on muscle regeneration and mitochondrial physiology during cancer-induced muscle wasting. This will contribute to the multimodal (exercise, nutrition and drug based) intervention strategies. We also aim to study the effects of adipose-induced muscle wasting, because also obesity can lead to muscle wasting. This is a result of inflammation of the muscle micro-environment caused by fat infiltration in the muscle. Therefore, we hypothesize that obesity in in combination with cancer can lead to more severe muscle wasting.
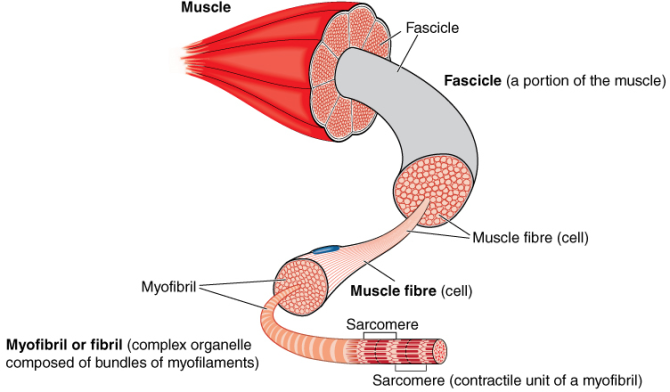
To study muscle regeneration and mitochondrial physiology a novel myofiber culture method will be validated and optimised to mimic the cancer- and adipose-induced changes in the muscle micro-environment. Different approaches will be used to investigate muscle regeneration and mitochondrial physiology including gene and protein expression analysis, fibertype composition, mitochondrial oxygen consumption and muscle stem cell activation. This will contribute to elucidating the pathways involved in muscle wasting. The myofiber contractility will be measured at Manchester Metropolitan University.