
Project
Novel methods for electrochemical capture and conversion of CO2
Climate change is one of the most critical global challenges. Increasing atmospheric CO2 concentration brought by anthropogenic emissions is the primary driver of climate change. Capturing CO2 from emission points and even directly from air provides a potential solution to mitigate the amount of CO2 emissions and reduce the atmospheric CO2 concentration. Anion exchange resin (AER), a polymeric material with amine-functionalized groups usually used in water desalination process, has been proven to be a promising solid amine sorbent for CO2 capture.
Technological challenge
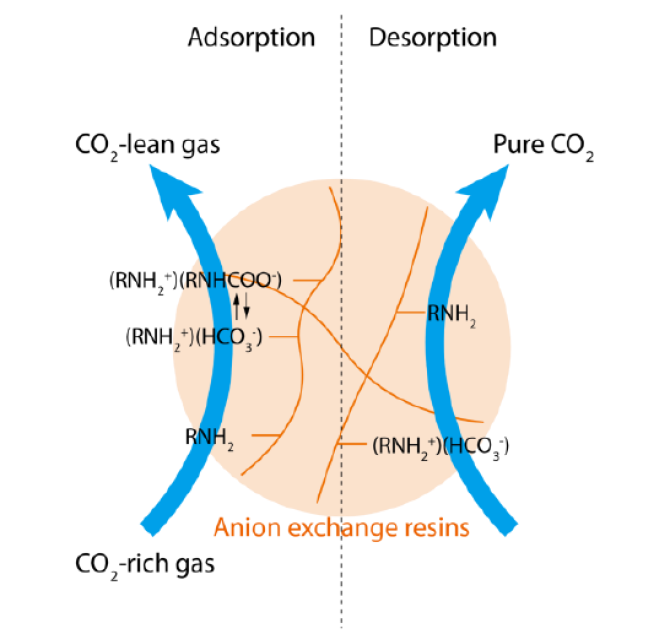
CO2 capture using ion exchange AERs involves adsorption and desorption steps. CO2 from CO2- rich stream can be adsorbed so that the outlet becomes CO2-lean stream, and pure CO2 can be produced during desorption. The adsorption process is due to the reaction between fixed amine groups on the resins and CO2. Since the reaction can be reversed at around 100 °C, conventional desorption process is by heating up the resins. Nevertheless, the regeneration of AERs using a high temperature restricts the application of this material owing to the high energy cost and likely degradation of resins.
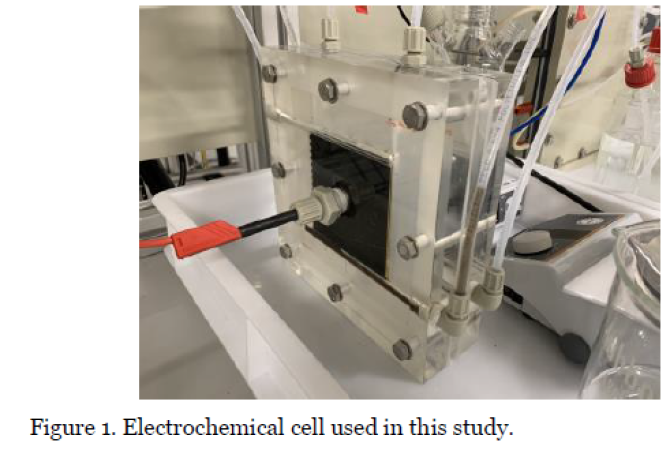
In this work, we propose a novel method to combine the conventional adsorption step with a more efficient electrochemical desorption step (Figure 1). The major challenge is to discover operation conditions that give high CO2 capture performance at low energy consumption.
Research goals
- Developing a novel system for CO2 capture based on ion exchange resins
- Investigating the CO2 capture performance and energy consumption of the system under different current density, CO2 partial pressure, and initial concentration of the regeneration solution
- Studying the performance of the system with different sorbents
- Developing a mathematical model of the system describing the kinetics and transport of various components