Project
Moderated ionic bonding for water-free recyclable polyelectrolyte complex materials
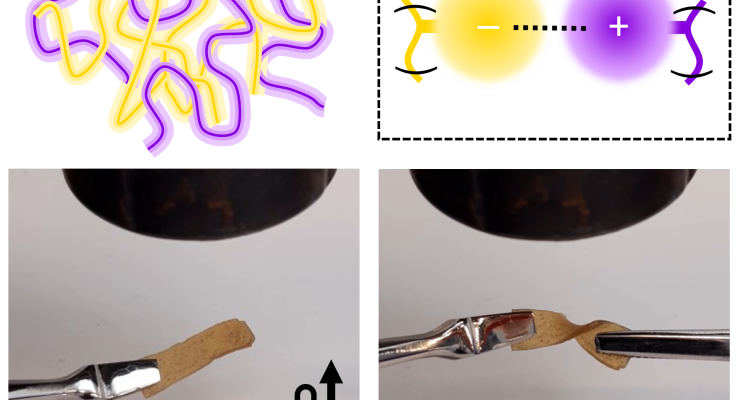
Nature makes abundant use of ionic interactions between oppositely charged macromolecules. In synthetic materials, the mechanics of such polyelectrolyte complexes (PECs) are highly dependent on their hydration level: ranging from liquid coacervates, to elastic PECs, to brittle and un-processable solids in the absence of plasticizing water. Controlling these attractive charges in dry conditions has proven to be a difficult challenge, and has prevented ionic interactions to be utilized as reversible crosslinkers in plastics.
We have successfully overcome these limitations, and developed a unique polymer class called ‘compleximers’, that uses the moderation of ionic interactions to form polyelectrolyte complex materials. We synthetically incorporate charge screening domains into polyelectrolytes by attaching bulky hydrophobic tails to the ends of charge-bearing side-chains. These bulky architectures weaken the ionic interactions, giving the so called ‘compleximers’ thermoplasticity, solvent-resistance and hydrophobicity. Compleximers thus marry the properties of thermoplastics and thermosets using tailored electrostatic bonding.