
Project
Process development of recombinant AAV manufacturing using the Baculovirus Expression System
Scalability is one of the main obstacles for the transfection-based manufacturing of pharmaceutical AAV vectors in mammalian cell lines. The usage of insect cell culture in combination with a Baculovirus Expression Vector System allows for the scalable recombinant AAV production needed for both clinical studies and commercial applications.
Using a viral vector to produce a viral vector
Gene therapy is a powerful technique that has a promising prospective for the treatment of many genetic diseases. The most effective tools to correct genetic defects are currently derived from viruses since they possess the inherent property to introduce their genetic material into host cells. The adeno-associated virus (AAV) is one of the most actively studied vectors for gene therapy. Recombinant AAVs, lacking the viral genes essential for replication, are capable of transducing various human tissues while being perceived as safe.
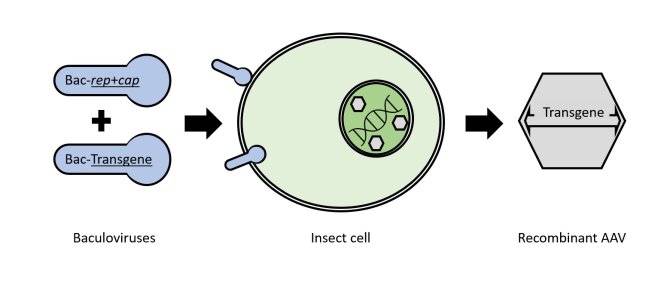
One of the main bottlenecks for therapeutic AAVs is the limited scale at which the particles can be manufactured when using transfection-based production in mammalian cell lines. One way to overcome the scalability issue is to use insect cell culture in combination with the Baculovirus Expression Vector System (BEVS). The BEVS is based on modified Baculoviruses that can infect insect cells leading to the production of recombinant proteins that are encoded in the Baculovirus genome. Scalability of BEVS is determined by the suspension based culture conditions in combination with replicative nature of the expression vectors. Recombinant AAV vectors harboring a therapeutic gene can be produced with BEVS by infecting insect cells with one or more specific Baculoviruses that contain the essential AAV genes (rep and cap) and the gene of interest (Transgene).
Although the BEVS-based production platform has already been used for several commercial AAV products, there are still major knowledge gaps concerning the insect cell metabolism and baculovirus infection kinetics. Additional research on these underexposed topics is essential for the rational development and intensification of the BEVS-based AAV production process.
Aim
This project aims to increase process understanding to support the logical design of a scalable upstream process for the manufacturing of therapeutic AAV particles using the Baculovirus Expression System.
Approach
In collaboration with the Laboratory of Virology and the gene therapy company VectorY, different molecular designs and bioprocessing strategies for recombinant AAV production using BEVS will be evaluated. At BPE, we will primarily focus on the development and characterization of a scalable upstream process as well as the implementation of improved Baculovirus constructs into the AAV vector manufacturing.
The initial step will be the set-up of a baseline upstream process for AAV production and to demonstrate the scalability. Continued development requires studying of the insect cell growth characteristics, infection kinetics and metabolism of the cells during cell growth versus baculovirus infection. The obtained knowledge on infection kinetics and metabolism will be integrated in process models to optimize volumetric productivity, product quality and reduce process variation. In addition, the use of online monitoring techniques will be implemented to increase overall process understanding.
Thesis project
If you are interested in this project or if you would like to write a BSc/MSc thesis on this topic, please do not hesitate to contact Mels Schrama.