Biosensing using Cholesteric Liquid Crystals
Liquid crystals (LCs) are a sort of “ordered fluid”: they flow like a liquid (an oil), but certain phases also possess an orientational ordering as a result of which the individual molecules prefer to point in a certain direction. A cholesteric, or a chiral nematic, LC, however, orders itself into a helical structure, giving rise to stunning reflected colors that are a function of its periodicity (due to Bragg reflection, Figure 1)1,2. This is very similar to the coloration we see in iridescent beetle shells3, butterfly wings, and certain kinds of plants4.
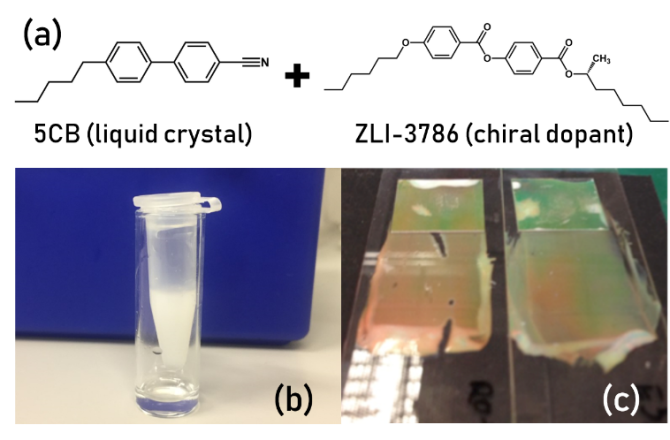
Classically, a cholesteric LC is created by using a “chiral dopant”, which is a chiral molecule structurally somewhat similar to the host, mixed into the LC5. Many natural materials, however, are naturally chiral, especially lipids, proteins, and other building blocks of the body. This brings up an interesting research question: can these chiral biomolecules also have an impact on the final chiral pitch and orientation of the LC? If yes, can we use their cholesteric properties for biosensing in order to detect specific macromolecules? The goal of this project is thus to investigate the appearance of reflected colors and structures in the presence of specific biomolecules (lipids, proteins, etc.) as a function of geometry and the LC material used. You will study this using flat LC films as well as via LC droplets and shells generated using on-chip microfluidics.
If you are interested in structural color, microfluidics, and biosensing, and if you want to work on an interdisciplinary project at the border between chemistry, soft matter physics, and biology, this project is ideal for you! Please contact Larry Honaker or Siddharth Deshpande for further details.
References
(1) St. John, W. D.; Fritz, W. J.; Lu, Z. J.; Yang, D.-K. Bragg Reflection from Cholesteric Liquid Crystals. Phys. Rev. E 1995, 51 (2), 1191–1198.
(2) Anyfantakis, M.; Jampani, V. S. R.; Kizhakidathazhath, R.; Binks, B. P.; Lagerwall, J. P. F. Responsive Photonic Liquid Marbles. Angew. Chemie - Int. Ed. 2020, 132, 2–10. https://doi.org/10.1002/ange.202008210.
(3) Agez, G.; Bayon, C.; Mitov, M. Multiwavelength Micromirrors in the Cuticle of Scarab Beetle Chrysina Gloriosa. Acta Biomater. 2017, 48, 357–367. https://doi.org/10.1016/j.actbio.2016.11.033.
(4) Vignolini, S.; Rudall, P. J.; Rowland, A. V.; Reed, A.; Moyroud, E.; Faden, R. B.; Baumberg, J. J.; Glover, B. J.; Steiner, U. Pointillist Structural Color in Pollia Fruit. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (39), 15712–15715. https://doi.org/10.1073/pnas.1210105109.
(5) Honaker, L. W.; Vats, S.; Anyfantakis, M.; Lagerwall, J. P. F. Elastic Sheath-Liquid Crystal Core Fibres Achieved by Microfluidic Wet Spinning. J. Mater. Chem. C 2019, 7 (37). https://doi.org/10.1039/c9tc03836a.